Abstract
Introduction Studies in patients with severe (HbSS and HbSβ0) sickle cell disease (SCD) have reported reduced oxygen metabolism compared to controls despite the preserved oxygen delivery (OD). Whereas previous studies have found similar lesion load in mild SCD patients (HbSC and HbSβ+), oxygen metabolism has not been studied in this group. Interestingly, we previously showed that CBF values in mild SCD patients were lower compared to severe SCD, and comparable with healthy controls. Here, we assessed the OD, oxygen extraction fraction (OEF), cerebral metabolic rate of oxygen (CMRO2) and lesion volumes in adult patients with severe and mild SCD and compared our results with thalassemia patients as anemic controls and healthy controls. In addition, the effect of a vasodilatory stimulus on the cerebral parameters was studied to test if increased CBF influences oxygen consumption.
Methods In this study, 75 patients with severe SCD (64 HbSS and 11 HbSβ0), 26 patients with mild SCD (16 HbSC and 10 HbSβ+), 17 patients with thalassemia (7 NTDT and 10 TDT) and 30 healthy controls were included. Venous blood T2 was measured using T2-Relaxation-Under-Spin-Tagging (TRUST). Whole brain (WB) CBF was measured using time-encoded pseudo-continuous ASL (te-ASL). TRUST and te-ASL scans were performed before and after acetazolamide (ACZ) administration as a vasodilatory stimulus. Fluid-Attenuated Inversion Recovery (FLAIR) scans were acquired for the assessment and segmentation of lesions. OEF was calculated from arterial saturation measured by pulse oximetry and venous saturation calculated from blood T2 using a sickle cell-specific calibration model and a healthy control model. CMRO2 was calculated from the WB CBF, OEF and oxygen content (calculated from hemoglobin and arterial saturation). To test the effect of ACZ-induced vasodilation, ∆CBF, ∆OD, ∆OEF and ∆CMRO2 were calculated. A one-way analysis of covariance (ANCOVA) was conducted (or a nonparametric alternative) with posthoc Bonferroni correction to test for statistically significant differences in cerebral parameters between the participating groups controlling for age and sex. For ∆CBF, ∆OD, ∆OEF and ∆CMRO2, baseline values were added as additional covariates.
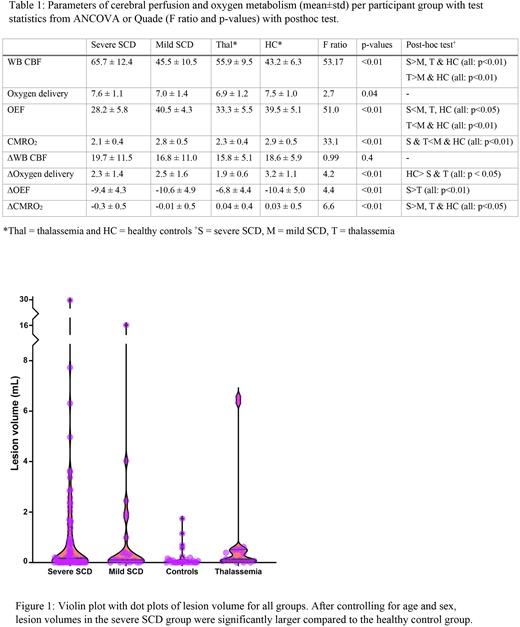
Results CBF was significantly higher in severe SCD compared to other groups and significantly higher in thalassemia patients compared to mild SCD and healthy controls (Table 1). No significant differences were found in OD between the groups. OEF was significantly lower in severe SCD patients compared to other groups and significantly lower in thalassemia patients compared to mild SCD patients and healthy controls (Table 1). CMRO2 was significantly lower in severe SCD and thalassemia patients compared to mild SCD patients and healthy controls (Table 1). ∆CBF upon ACZ administration was not significantly different between the groups. ∆OD was significantly higher in controls compared to severe SCD and thalassemia patients (Table 1). Infusion of ACZ impaired the OEF and CMRO2 more in severe SCD compared to the other groups (Table 1: ∆OEF and ∆CMRO2). Lesion volume was significantly larger in the severe SCD group compared to the healthy controls but no significant differences were found between the other groups (Fig. 1).
Conclusion Our results show that OEF and CMRO2 were significantly higher in mild SCD patients, and no different from healthy controls, compared to severe SCD and thalassemia patients, illustrating a more balanced oxygen supply versus demand in these less anemic patients. Furthermore, the finding that ACZ-induced elevated CBF significantly reduced the CMRO2 in severe SCD, but not in the other groups, suggests that functional shunting may play a role in severely affected SCD patients. Despite the large differences in cerebral oxygen metabolism between severe and mild SCD, no difference in lesion volume could be observed between these groups. One possible mechanism that might be responsible for the lesion load in mild SCD patients could be the higher blood viscosity in this group that has been hypothesized to cause other complications such as retinopathy, particularly in patients with HbSC. Another explanation could be that lesion volume is more related to deep brain hypoxia than whole brain oxygen metabolism which could be confirmed by further studies investigating deep brain oxygenation.
Disclosures
Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Biemond:Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Sanquin: Research Funding; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal